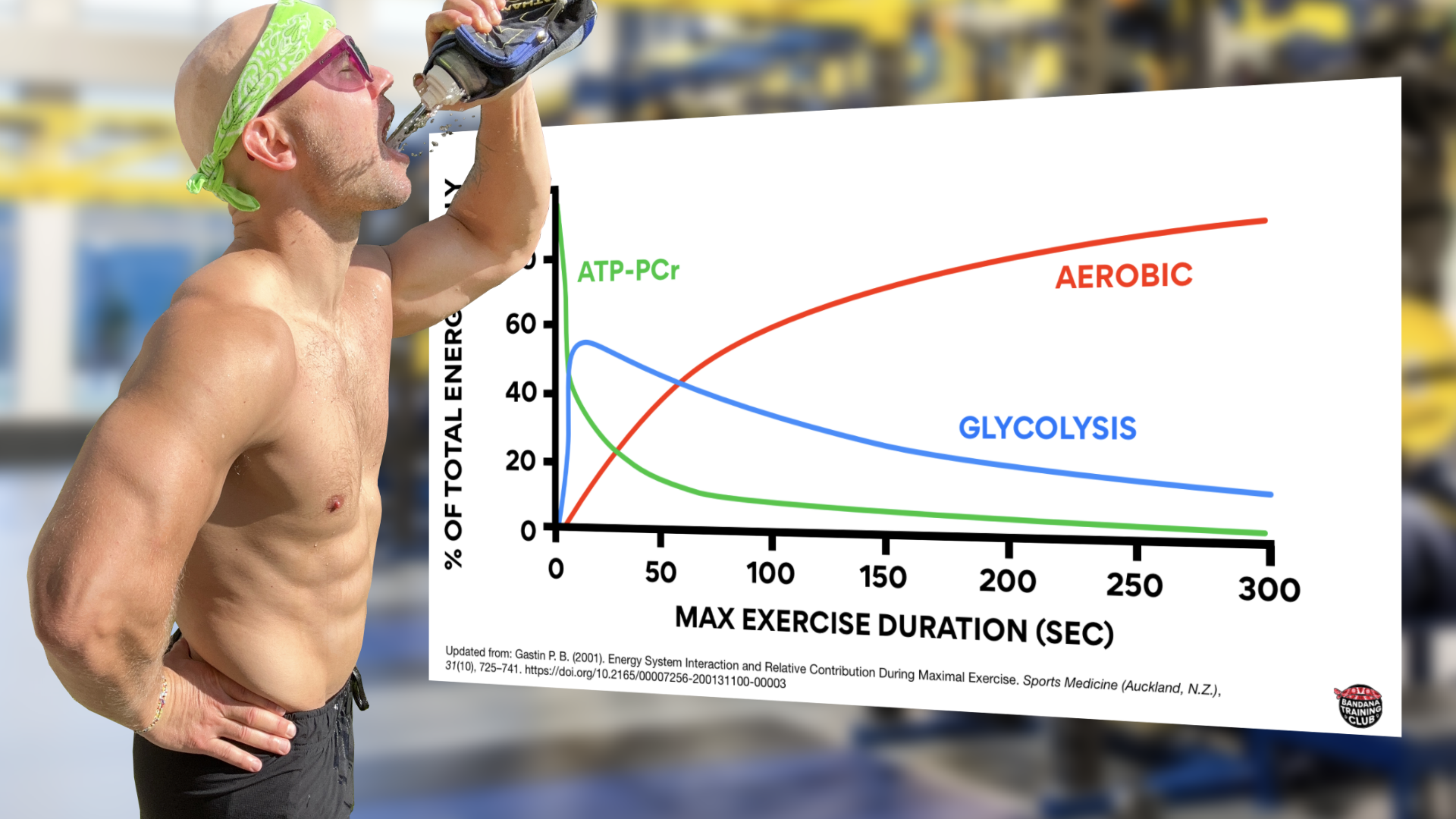
Humans have three primary energy systems – the creatine phosphate system, anaerobic glycolysis, and our aerobic system. The first two don’t require oxygen and can produce energy rather quickly while the aerobic system, as the name implies, requires oxygen but produces energy relatively slowly. We’re going to explore the nuts-and-bolts of each of these systems but before we get into the biochemistry, it’s worth asking…why do we care?
Why is it helpful for coaches and athletes to understand bioenergetics?
When we start discussing chemical equations, it’s reasonable to get a lil’ bleary-eyed and wonder how adenosine triphosphate is going to help athletes win championships. After all, energy systems don’t control the line of scrimmage or exploit a weak secondary, athletes do. But successful coach and athletes are always looking for ways to deconstruct and explain complex systems. From a biological perspective, that’s exactly what we’re doing. We’re deconstructing the components that help athletes produce energy which is at the foundation of their success. A more profound understanding of these systems ultimately allow us to create protocols and interventions that optimize them.
Energy Production 101
ATP – The Whole Shebang
Adenosine Triphosphate (ATP) is the universal currency of energy in our body. All living cells use ATP as fuel (take a second to ponder that.) In humans, each cell contains roughly 1 billion ATP molecules, all of which will be used and replaced every 2 minutes, even at rest (Meyer and Wiseman 2012). The magnitude and complexity of this is hard to wrap our head around, although we’ll do our best.
The goal of our energy systems is to produce ATP. ATP is adenosine triphosphate – it’s an adenosine backbone with 3 phosphate groups attached. These 3 phosphate groups are linked together by high-energy bonds, so breaking off a phosphate group releases energy. We break down adenosine triphosphate into adenosine diphosphate (2 phosphate groups). We call this processes hydrolysis because it requires water. Here’s the chemical equation:
ATP + H20 ===> ADP + Pi (inorganic phosphate) + energy
Because we’re always breaking down ATP into ADP to produce energy, we need to constantly replace this ATP. To do this, we put our thing down flip it and reverse it (was that a Missy Elliott reference when discussing biochemistry?)
ADP + Pi (inorganic phosphate) + energy ===> ATP + H20
As you can see, it’s the same equation in reverse; we need inorganic phosphate and energy to reproduce ATP. The quickest and simplest way for us to get Pi and energy is with our creatine phosphate system.
Creatine Phosphate
Creatine phosphate (CP), also called phosphocreatine (PCr), is a creatine molecule with a phosphate attached. Remember, the star of our show is adenosine triphosphate so as you might imagine from the name, creatine phosphate is able to generously donate its phosphate molecule to reproduce ATP.
Here’s the simple chemical equation:
PCr + ADP ===> ATP + Cr
This process is pretty straightforward and produces a single ATP molecule for every cycle. PCr is stored in our muscle cells, which makes this process quick, simple, and locally sourced. Here’s the problem: we can’t store much PCr in our muscles, although supplementing with creatine monohydrate can help. After about 20 seconds of max effort this system is tapped out at which point our next anaerobic energy system has already stepped up. (Gastin, 2001)
Anaerobic Glycolysis
Let’s start with the phrase itself: anaerobic glycolysis. Anaerobic means “without oxygen” and any word that ends with “lysis” means “breaking it down,” so anaerobic glycolysis means breaking down glucose without oxygen.
Glucose is a carbohydrate. Again, the word “carbohydrate” gives us a nice clue as to what it is. A carbo-hydrate is carbon that has been hydrated. Glucose is almost always a 6 carbon molecule, making the chemical formula C6H12O6. In order to break down glucose, we start by snapping it in half to form two 3 carbon molecules called pyruvate. This process requires 2 ATP molecules, but it produces 4 so we end up 2 net positive. It’s a slightly more complicated chemical process that looks like this:
Glucose + 2 NAD+ + 2 ADP + 2 Pi ===> 2 Pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
Here’s when things get wild because now we have a metabolic choice to make. If we need energy quickly and there isn’t enough oxygen present, we take this newly-created pyruvate and the excess hydrogen and attach them together.
Pyruvate + Hydrogen = Lactate
All the while, we’re gaining 2 molecules of ATP by breaking glucose down into pyruvate then slapping hydrogen on to produce lactate. As lactate starts to accumulate, we shuttle lactate around and can actually use it as a fuel source in other muscle fibers, including the heart, to produce energy. This system works pretty well for about 1 minute, at which point our aerobic system has finally gathered some oxygen and enters the scene. (Brooks, 2018; Chaudhry & Varacallo, 2023; Gastin, 2001)
This is the first of a two part series on energy systems. In Part 2, we’ll explore our aerobic system which is the powerhouse of energy production and how to practical apply this science.
If you’re learning from these posts, please follow me on social media (links below) and consider signing up for the Bandana Training Club which contains an entire catalogue of training programs and sport science related content.
Works Cited
Brooks G. A. (2018). The Science and Translation of Lactate Shuttle Theory. Cell Metabolism, 27(4), 757–785. https://doi.org/10.1016/j.cmet.2018.03.008
Chaudhry R, Varacallo M. (2023). Biochemistry, Glycolysis. In: StatPearls. StatPearls Publishing, Treasure Island (FL); PMID: 29493928.
da Costa, C., & Galembeck, E. (2016). The Evolution of the Krebs cycle: A Promising Subject for Meaningful Learning of Biochemistry. Biochemistry and Molecular Biology Education : a Bimonthly Publication of the International Union of Biochemistry and Molecular Biology, 44(3), 288–296. https://doi.org/10.1002/bmb.20946
Gastin P. B. (2001). Energy System Interaction and Relative Contribution During Maximal Exercise. Sports Medicine (Auckland, N.Z.), 31(10), 725–741. https://doi.org/10.2165/00007256-200131100-00003
Lane, Nick. (2022). Transformer: The Deep Chemistry of Life and Death. W. W. Norton & Company. ISBN: 0393651487.
Meyer R.A. & Wiseman R.W. (2012). The Metabolic Systems: Control of ATP Synthesis in Skeletal Muscle. ACSM’s Advanced Exercise Physiology. 2nd ed. Philadelphia, PA: Wolters Kluwer, 363–378.
Peek, C. B., Levine, D. C., Cedernaes, J., Taguchi, A., Kobayashi, Y., Tsai, S. J., Bonar, N. A., McNulty, M. R., Ramsey, K. M., & Bass, J. (2017). Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metabolism, 25(1), 86–92. https://doi.org/10.1016/j.cmet.2016.09.010